Medical Industry - Polarion ALM

Use Polarion ALM to Achieve Engineering Excellence
Overview
How Polarion ALM is used across industries helps to improve System, Software, Hardware and Mechatronics development, specification, testing and management.
ALM - Application Lifecycle Management requires one single tool covering multiple areas of product development.
Achieve Compliance with different standards by using Polarion ALM. Automate Proof of Compliance.

|
- IEC 62304
- FDA 21 CFR Part 820
- FDA 21 CFR Part 11
- ISO 14971:2012 / ISO 14971:2007
|
|
Key Words & Facts: compliance, FDA
#2 Full control & history of changes
|
Electronic signatures can be used to sign Documents and individual items. Every "save" operation is visible in history logs.
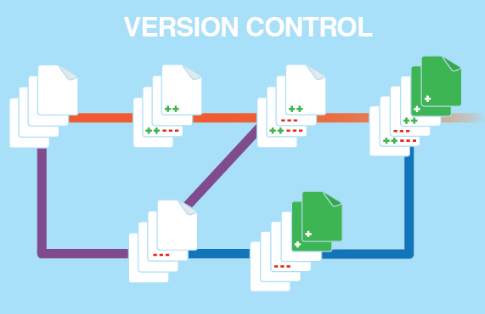
|
- Version Control system "SVN"
- Full History of Changes
- works & is available "by default"
|
|
Key Words & Facts: compliance, FDA
Polarion has great features for creating Links between items. This allows to trace the links from one item to another and to create Traceability Tables.
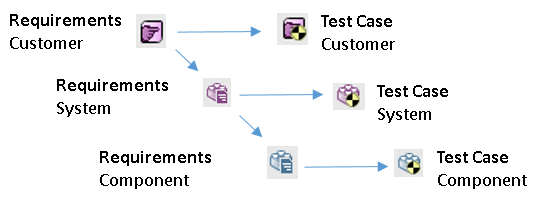
|
- Traceability from User Story to Requiremnts and down to Source Code;
- Built-in support for ReqIF data exchange, and/or through common document formats such as Word/Excel® or PDF®;
- Seamless real-time collaboration, contextual visibility improves team effectiveness and intuitive workflow drives rapid adoption;
- Comprehensive traceability and automated tracking speeds and protects functional safety compliance;
- Model Driven development with Polarion’s Diagram Editor, plus integration with Sparx Systems Enterprise Architect™ and with MATLAB® Simulink™;
- Enabling automotive industry project templates facilitate functional safety compliance, process assessment, Agile development practices, and other objectives
|
|
V-model Traceability
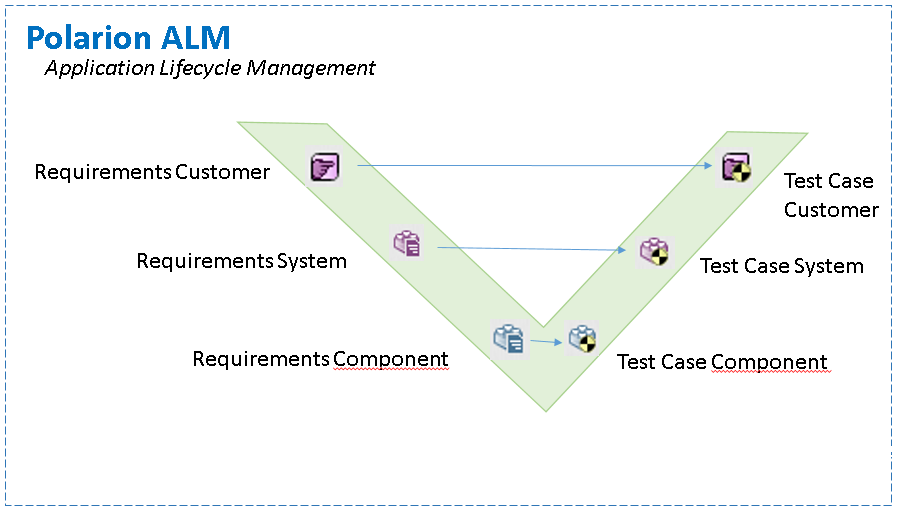
Traceability Matrix
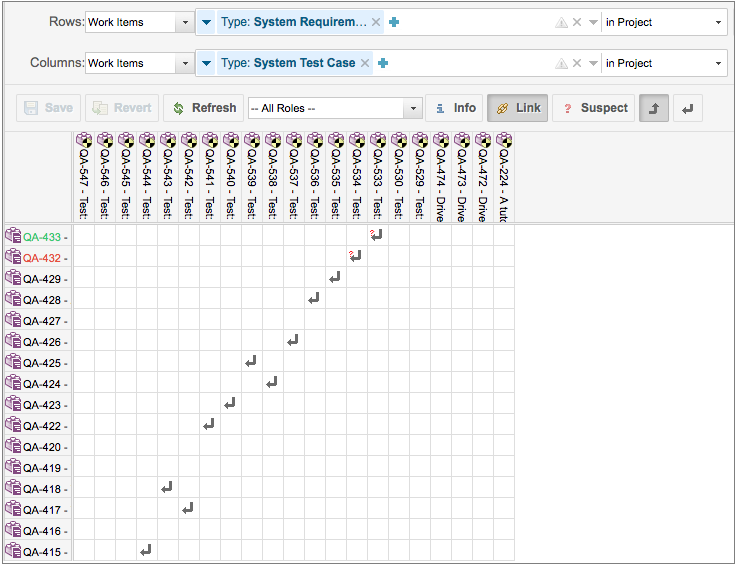
Requirement to TestCase Traceability
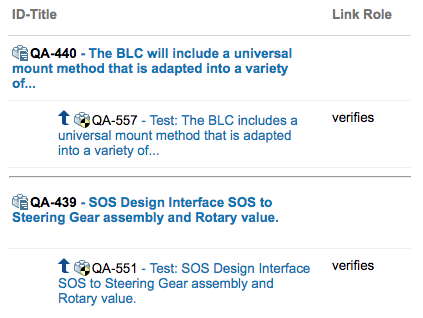
Key Words & Facts: ISO 26262, Automotive SPICE and CMMI
Enterprise Agile Software Development Solution Details

|
- Full Traceability from Request to Implementation: review the impact of planned or implemented change in single click.
- Baselining & Versioning: Agile means change. In Polarion you can quickly see who changed what, when, and why!
- Fast Start: Import your existing data from Microsoft® Word or Excel® to create your Product Backlog online, in minutes.
- Flexible Live Reporting with out-of-the-box sprint and release burn-down charts, kanban boards, total cost/estimate aggregation, velocity calculation,
plus an entire portal of other reports and extensions. Everything can be tailored to your data model.
- Collaboration for Distributed Agile Teams: No out-of-sync emails, all artifacts in one place and always up to date, social media-like project activity streams keep everyone informed.
- Collaboration in Hybrid Process Scenarios use Polarion's exclusive Round-trip features to share and re-import data with external contributors and Scrum teams.
- Cross-Project Reporting and Dashboards: view the actual status of all your running projects, search for issues or enhancements across multiple projects.
Anything you can find out for 1 project you can find across several.
- Subversion & Git: Integrates with Subversion and Git out of the box. Link changes from within IDEs like Eclipse for ultimate convenience creating traceability.
(Do you know that Polarion is an Eclipse Foundation Member, and we manage the Subversive project?)
|
|
Key Words & Facts: Full Traceability, Baselining & Versioning, Fast Start, Live Reporting, Collaboration, Cross-Project, Kanban, Scrum board.

Risk identification/review, assessmen and recording. Individual risks managed by identified risk owners.
Polarion helps customers automate systems engineering and develop quality embedded software with the industry's highest efficiency and ROI.
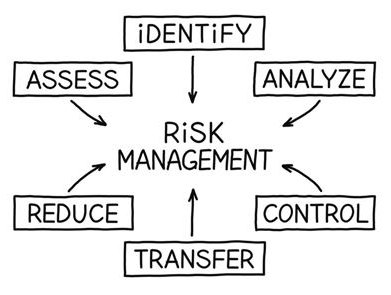
|
- Identify Risk
- Assess Risk
- Analyze Risk
- Control Risk
- Review Controls
- Reduce Risk
|
|
Template for Risk Management
Polarion has a predefined Template to perform Risk Management and to establish traceabilty between Requirements, Risks and Risk Reduction Measures.
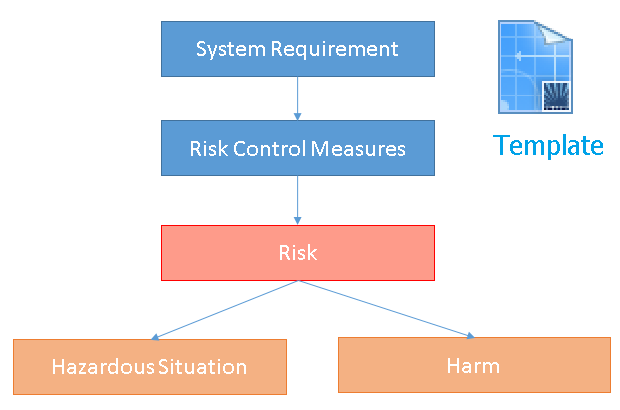
|
- contains items System/Software/Hardware Requirments
- contains links definition between items
- contains logic and reports for analysis of risks
- contains traceability tables for risk overview
- is re-configurable
- is documented
|
|
Key Words & Facts: Agile, Waterfall, V-Model, ISO/IEC, FDA, FAA, FMEA, Code traceability.
The challenges for embedded development teams and device manufacturers is to provide as much functionality as possible in new and differentiated products,
while still meeting aggressive demands on price, size, performance, and time to market.
Polarion helps customers automate systems engineering and develop quality embedded software with the industry's highest efficiency and ROI.

|
- Real-time collaboration with intuitive authoring and linking to quickly identify defects, determine impact
- Comprehensive end-to-end traceability from requirements to code, testing, verification and validation
- Customizable, pre-built best-practice workflows, cross-discipline reports, and metrics to manage the full product lifecycle
- Predictive analysis, FMEA risk management and lifecycle project management
- Use your own existing processes or the ramp fast using expert templates provided for Agile, Waterfall, V-Model, ISO/IEC, FDA, FAA, and hundreds more<
- Managing requirements, code, test cases, work items of any kind for product variants
- Track, Trace, or Link any kind of work items one-to-one or one-to-many
|
|
Key Words & Facts: Agile, Waterfall, V-Model, ISO/IEC, FDA, FAA, FMEA, Code traceability.
#7 Standards Support - Compliance in Details
|
Read in details how you can be compliant with different standards with help of Polarion ALM.
ISO 14971:2012
ISO 14971:2012 is necessary when manufacturer sells inside and outside Europe.
The ISO 14971:2012 that is necessary in Europe and old one ISO 14971:2007 still applies to all areas outside of Europe.
With the help of polarion you can easily achieve both standards and always see the difference with a click of the mouse.
IEC 62304
Polarion helps to achieve compliance with this Standard.
FDA 21 CFR Part 820
Polarion helps to achieve compliance with this Standard.
FDA 21 CFR Part 11
Polarion helps to achieve compliance with this Standard.
Key Words & Facts: Standards.